
Atomic number,mass number and valency of elements 1 to 30 YouTube
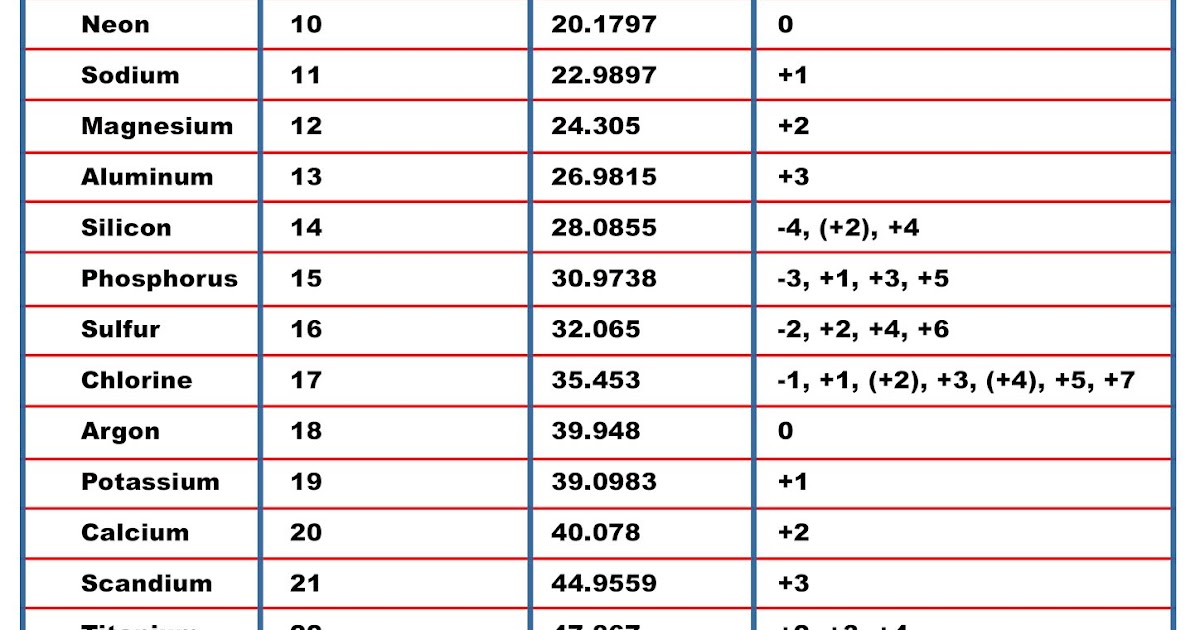
Here is a table of element valences. Remember that an element's electron cloud will become more stable by filling, emptying, or half-filling the shell. Also, shells don't stack neatly one on top of another, so don't always assume an element's valence is determined by the number of electrons in its outer shell. Table of Element Valences Sources

A simple way to understand and memorise the valency of first 20 elements of the periodic table
In less than 2 minutes, you'll remember the valency of elements 1 - 30! We'll use 2 valency tricks - valency for the first 20 elements and valency for elemen.
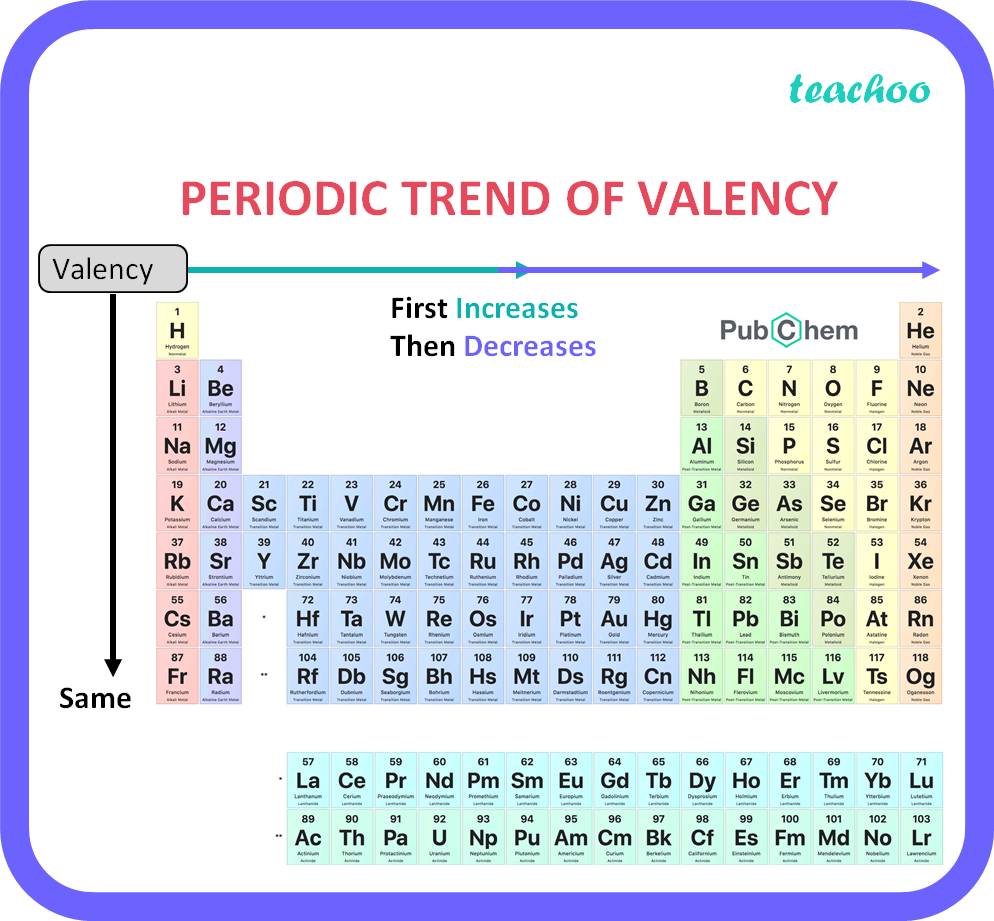
How does valency of an element vary across a period Class 10 Teachoo
The valence (or valency) of an element is a measure of its combining power with other atoms when it forms chemical compounds or molecules. The concept of valence was developed in the last half of the 19th century and was successful in explaining the molecular structure of many organic compounds. The quest for the underlying causes of valence.
Periodic Table And Their Valency
Q1What are the first 20 elements in order?H - HydrogenHe - HeliumLi - LithiumBe - BerylliumB - BoronC - CarbonN - NitrogenO - OxygenF - FluorineNe - NeonNa -.
Valency and Variable Valency Valence Shell and Electrons » Selftution
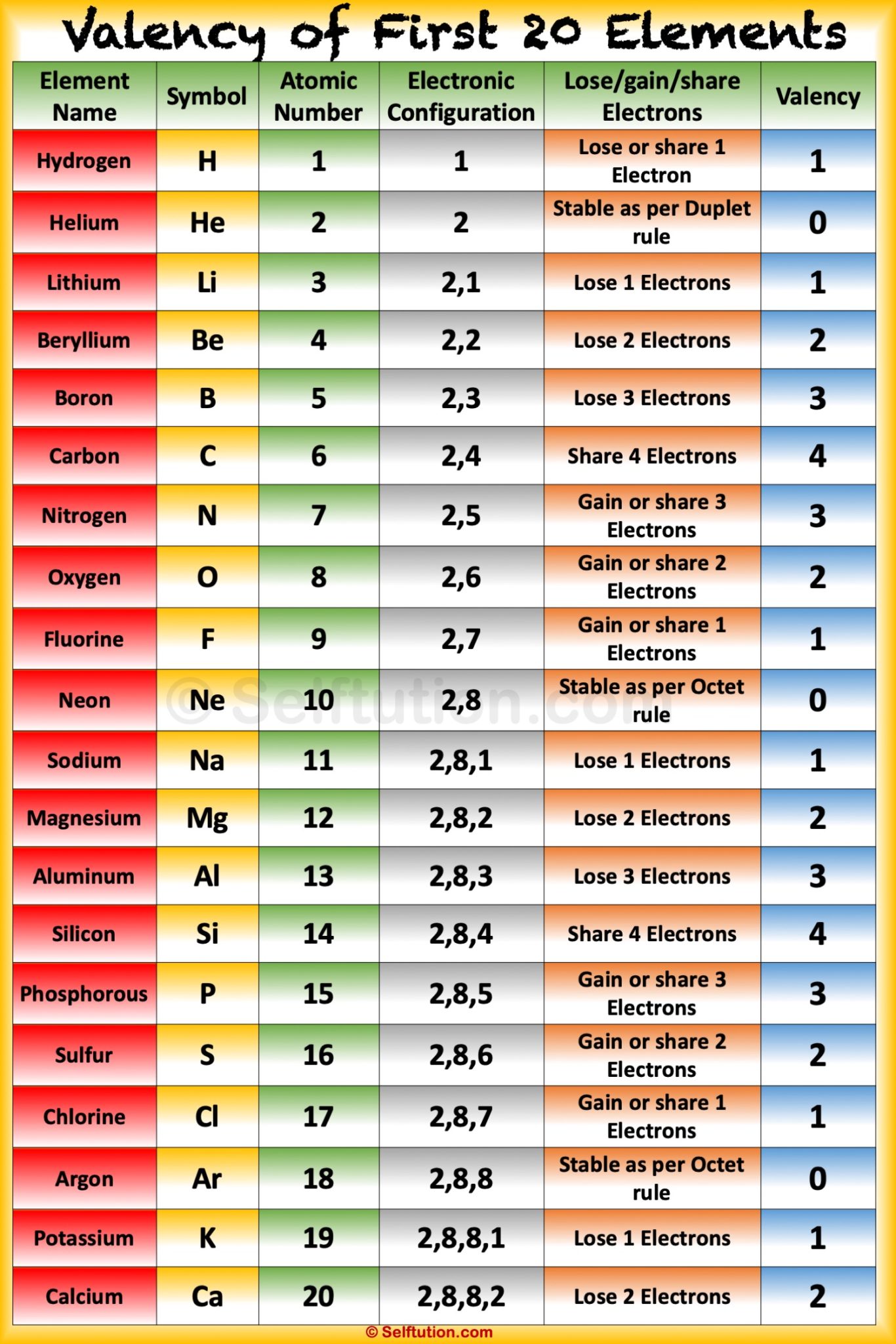
First 20 Elements The first 20 elements of the periodic table have been tabulated below, along with their symbols and atomic numbers. Learn more ⇒ Interactive Periodic Table Table of Contents What Information does the Atomic Number of an Element Provide? Why is Potassium denoted by the symbol 'K' and Sodium by the symbol 'Na'? Recommended Videos

Periyodik tablo Değerlik Kimyasal element Kimya, periyodik, kimyasal element, açı, mobilya png
An atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . The number of valence electrons determines most of an atom's chemical behaviors. So, it's important to be able to identify how many valence electrons atoms of different elements have. Fortunately, we can.

chemistry first 20 elements table with electronic configuration and valency Brainly.in
Solution Valency:- Valency is defined as the property of an element in which it has the combining capacity with another atom of an element. It is related to electrons present in their outer orbital. Valency can be determined by their respective groups in the periodic table. Determination of valency of first 20 elements:-

valency trick how to find valency of elementsfirst 20 elements YouTube
Element Valency PDF. The outer shell of a fluorine atom contains 7 electrons. This means it has one less electron than needed to complete the shell. This gives fluorine a -1 valence. This element Valency PDF is a downloadable version of the Valences of the Elements table. As in the table, the most common valences are in BOLD text where values.

Electronic Configuration Definition, Shell, Position
How are these derived? The chemical formula of any element is written because of the valencies of its compound. In the following article we are going to discuss the answer to these questions which is 'Valency' , its examples and uses. What does the term Valency mean? Valency is the combining capacity of an element. It is always a whole number.

First 30 Elements Of Periodic Table With Valency
The valence electrons for main group elements are those with the highest n level. For example, gallium (Ga, atomic number 31) has the electron configuration [Ar]4s 2 3d 10 4p 1, which contains three valence electrons (underlined - 4s 2, 4p 1). The completely filled d orbitals count as core, not valence, electrons. Transition elements or.

20 elements name symbol. valency atomic mass atomic number Brainly.in
Valence electrons: For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.

periodic table of elements printable flashcards chemistry etsy find various types of valency
Hence we assign a valence of 1 to H and to Cl. The valence of O is twice as great, and so we assign a value of 2. Example 4.4.1 4.4. 1: Formula Predictions. Use the data in the first table to predict what formula would be expected for a compound containing (a) sodium and fluorine; (b) calcium and fluorine.

First 20 Elements Of The Periodic Table With Atomic Number And Mass Valency Review Home Decor
1. Variation Of Oxidation State Along a Period While moving left to right across a period, the number of valence electrons of elements increases and varies between 1 to 8. But the valency of elements, when combined with H or O first, increases from 1 to 4 and then it reduces to zero. Consider two compounds containing oxygen Na 2 O and F 2 O.

what is the valency of first 20 elements ? Brainly.in
Updated on September 30, 2018. The words valence and valency have two related meanings in chemistry. Valence describes how easily an atom or radical can combine with other chemical species. This is determined based on the number of electrons that would be added, lost, or shared if it reacts with other atoms. Valence is denoted using a positive.

valency table Scribd india
Description The valence is the combining capacity of an atom of a given element, determined by the number of hydrogen atoms that it combines with. In methane, carbon has a valence of 4; in ammonia, nitrogen has a valence of 3; in water, oxygen has a valence of 2; and in hydrogen chloride, chlorine has a valence of 1.

1 to 30 elements with valency electrons Brainly.in
Solution Valency: "The electrons present in the outermost shell of an atom are known as the valence electrons." "Valency is the combining capacity of an atom." The valency and valence electrons for the first 20 elements are discussed below: Suggest Corrections 953 Similar questions